+86-18186629601
- All
- Product Name
- Product Keyword
- Product Model
- Product Summary
- Product Description
- Multi Field Search
| Availability: | |
|---|---|
4-Chlorobenzophenone CAS 134-85-0 Product Information
| CAS No | 134-85-0 |
| Product Name | 4-Chlorobenzophenone |
| Synonyms | 2-BROMO-1-(4-CHLOROPHENYL)ETHAN-1-ONE;chlorobenzophenone;Photoinitiator CBP;4-CHLOROPHENACYL BROMIDE;p-CBP;AKOS 90589;IHT-PI CBP;AKOS BBS-00000813;4-Dichlorophenone;Lencolo 5039(CBP) |
| Free Sample | Available |
| Molecular Formula | C13H9ClO |
| Molecular Weight | 216.66 |
| Purity | 99% |
| Free Shipping | YES |
4-Chlorobenzophenone CAS 134-85-0 Product Information
| CAS No | 134-85-0 |
| Product Name | 4-Chlorobenzophenone |
| Synonyms | 2-BROMO-1-(4-CHLOROPHENYL)ETHAN-1-ONE;chlorobenzophenone;Photoinitiator CBP;4-CHLOROPHENACYL BROMIDE;p-CBP;AKOS 90589;IHT-PI CBP;AKOS BBS-00000813;4-Dichlorophenone;Lencolo 5039(CBP) |
| Free Sample | Available |
| Molecular Formula | C13H9ClO |
| Molecular Weight | 216.66 |
| Purity | 99% |
| Free Shipping | YES |
4-Chlorobenzophenone CAS 134-85-0 Chemical Properties
| Melting point | 74-76 °C (lit.) |
| Boiling point | 195-196 °C/17 mmHg (lit.) |
| Density | 1.1459 (rough estimate) |
| Vapor Pressure | 0.015Pa at 25℃ |
| Refractive index | 1.5260 (estimate) |
| Flash point | 143°C |
| Storage conditions | 2-8°C |
| Storage temp | Sealed in dry,Room Temperature |
| Solubility | Chloroform (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly) |
| Acidity coefficient (pKa) | 3.03±0.10(Predicted) |
| Form | powder |
| Color | White to off-white |
| Water solubility | 20.706mg/L at 29℃ |
BRN | 6354 |
| InChIKey | UGVRJVHOJNYEHR-UHFFFAOYSA-N |
| LogP | 3.748 at 25℃ |
4-Chlorobenzophenone CAS 134-85-0 Chemical Properties
| Melting point | 74-76 °C (lit.) |
| Boiling point | 195-196 °C/17 mmHg (lit.) |
| Density | 1.1459 (rough estimate) |
| Vapor Pressure | 0.015Pa at 25℃ |
| Refractive index | 1.5260 (estimate) |
| Flash point | 143°C |
| Storage conditions | 2-8°C |
| Storage temp | Sealed in dry,Room Temperature |
| Solubility | Chloroform (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly) |
| Acidity coefficient (pKa) | 3.03±0.10(Predicted) |
| Form | powder |
| Color | White to off-white |
| Water solubility | 20.706mg/L at 29℃ |
BRN | 6354 |
| InChIKey | UGVRJVHOJNYEHR-UHFFFAOYSA-N |
| LogP | 3.748 at 25℃ |
4-Chlorobenzophenone CAS 134-85-0 Chemical Properties,Uses,Production
Application
4-Chlorobenzophenone is an off-white or off-white to reddish-white crystal.It is a raw material for the synthesis of pharmaceutical and pesticide products such as the anti-hyperlipidemic drug fenofibrate and the preparation of heat-resistant polymers. It has a wide range of uses. In addition, 4-chlorobenzophenone, as an important chemical intermediate, is widely used in medicine, pesticides, dyes and other organic synthesis.
Resolve Resolution
Combine 4-chlorobenzoyl chloride (1.5mmol), phenylboronic acid (1.25mmol), and sodium carbonate (133mg, 1.25mmol). Flush the tube with argon.Seal the tube with a rubber septum. Add toluene and water (3 each) to the test tube. mL). Transfer the reaction vessel to an oil bath maintained at 50 °C.Stir the mixture for 1 h. Transfer the reaction mixture to a separatory funnel. Dilute the mixture with diethyl ether (20 mL). Separate the aqueous phase. Separate the aqueous phase with 3 M HCl ( twice), 5% KOH (four times) and brine (twice).Dry the organic phase over anhydrous magnesium sulfate. Pre-adsorb the crude product by evaporating the organic phase under vacuum over chromatography grade silica gel.Use hexane-ethyl acetate The product was purified by column chromatography on silica gel (30:1, 10:1 or 5:1 v:v). The product was evaporated to give the title compound 4-chlorobenzophenone.
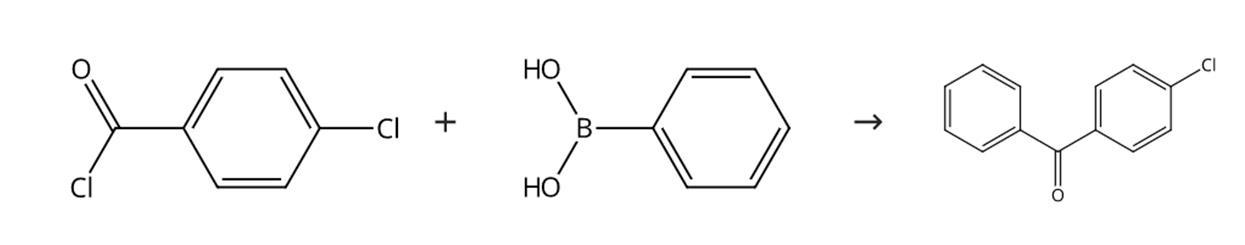
Figure 4-Synthetic route of chlorobenzophenone
Chemical Properties
Off-white crystalline powder
Use
UV curable coatings and inks
Use
Pharmaceutical intermediates
Use
Used as pharmaceutical and pesticide intermediates
Production Method
Obtained from the condensation of benzoyl chloride and chlorobenzene: In a dry reaction pot, add chlorobenzene and anhydrous aluminum trichloride,stir and raise the temperature to 50°C, and add benzoyl chloride dropwise. After the addition, react at 100-110°C for 5.5 hours, slowly add slightly acidic ice water, stir well and let it stand, separate the supernatant, filter, wash until neutral, and spin dry to obtain 4-chlorobis benzophenone.
4-Chlorobenzophenone CAS 134-85-0 Chemical Properties,Uses,Production
Application
4-Chlorobenzophenone is an off-white or off-white to reddish-white crystal.It is a raw material for the synthesis of pharmaceutical and pesticide products such as the anti-hyperlipidemic drug fenofibrate and the preparation of heat-resistant polymers. It has a wide range of uses. In addition, 4-chlorobenzophenone, as an important chemical intermediate, is widely used in medicine, pesticides, dyes and other organic synthesis.
Resolve Resolution
Combine 4-chlorobenzoyl chloride (1.5mmol), phenylboronic acid (1.25mmol), and sodium carbonate (133mg, 1.25mmol). Flush the tube with argon.Seal the tube with a rubber septum. Add toluene and water (3 each) to the test tube. mL). Transfer the reaction vessel to an oil bath maintained at 50 °C.Stir the mixture for 1 h. Transfer the reaction mixture to a separatory funnel. Dilute the mixture with diethyl ether (20 mL). Separate the aqueous phase. Separate the aqueous phase with 3 M HCl ( twice), 5% KOH (four times) and brine (twice).Dry the organic phase over anhydrous magnesium sulfate. Pre-adsorb the crude product by evaporating the organic phase under vacuum over chromatography grade silica gel.Use hexane-ethyl acetate The product was purified by column chromatography on silica gel (30:1, 10:1 or 5:1 v:v). The product was evaporated to give the title compound 4-chlorobenzophenone.

Figure 4-Synthetic route of chlorobenzophenone
Chemical Properties
Off-white crystalline powder
Use
UV curable coatings and inks
Use
Pharmaceutical intermediates
Use
Used as pharmaceutical and pesticide intermediates
Production Method
Obtained from the condensation of benzoyl chloride and chlorobenzene: In a dry reaction pot, add chlorobenzene and anhydrous aluminum trichloride,stir and raise the temperature to 50°C, and add benzoyl chloride dropwise. After the addition, react at 100-110°C for 5.5 hours, slowly add slightly acidic ice water, stir well and let it stand, separate the supernatant, filter, wash until neutral, and spin dry to obtain 4-chlorobis benzophenone.